Lupine Publishers Pediatrics and Neonatology
Abstract
Background: Gastroenteritis, caused by either bacteria, virus, or parasites, is a gastrointestinal inflammatory illness known by many. Lactoferrin (Lf), a protein primary found in human milk, is released from activated polymorphonuclear leucocytes during an inflammatory response. As such, Lf could be suggested as an indicator for the degree of inflammation in gastroenteritis.
Objective: We aimed to investigate the degree of inflammation by the level of fecal Lf compared to the culture of well-known bacterial enteric pathogens in toddlers and adults, and in toddlers to examine if the intake of breast milk was associated with higher levels of fecal Lf when compared to non-breastfed toddlers.
Methods: Levels of fecal Lf and fecal bacterial culture were analyzed in 512 consecutive fecal samples from patients with diarrhea where physicians found sampling indicated. Further, to assess the number of toddlers who had breast milk, the medical records were obtained from the National Diagnostic Register.
Results: 477 out of 512 patients (93%) with gastrointestinal symptoms had positive fecal cultures with the most common bacteria being Clostridum difficile 263 (51%). In patients between 3 and 94 years of age, all Shigella sonnii and shiga-toxin producing Escherichia coli indicated severe inflammation with fecal Lf levels >10,000 pg/ml, although only Shigella was significant when compared with normal young adults with fecal Lf levels < 100 pg/ml and without gastrointestinal symptoms (controls) (p = 0.0002). All other bacteria-positive feces cultures were associated to increased levels of fecal Lf > 1,000 pg/ml when compared with controls (p < .05). In patients between 3 weeks and less than 3 years of age, all pathogenic bacteria cultured, apart from Salmonella enteritidis and Clostridium perfringens (enterotoxin positive), were significantly associated with fecal Lf levels > 1,000 pg/ml when compared with controls (p <.05). The severity of gastrointestinal symptoms and levels of fecal Lf were not significantly associated (p-value 1.0). Breast-milk (partially and fully) vs formula-milk and fecal Lf levels in toddlers < 6 months of age revealed no significant differences (p = 0.29) Conclusion: High levels of fecal Lf were associated with positive cultures of feces, and the more aggressive the bacteria cultured was, the higher level of fecal Lf was observed, when compared with normal levels of Lf in young healthy men and women without gastrointestinal symptoms. In an unmatched association-analysis, no significant differences between breast milk and formula milk concerning fecal Lf levels were observed in our study.
Keywords:Lactoferrin; Bacterial Enteric Pathogens; Gastroenteritis; Necrotizing Enterocolitis; Diarrhea
Introduction
Lactoferrin, (Lf), a member of the transferrin family of non-haem iron-binding proteins, is a compound found primarily in milk and mucosal secretions [1]. Lf is considered a multifunctional protein, mainly involved in both the innate and adaptive immune defenses of the organism and is released from activated polymorphonuclear leucocytes (PMN’s) during an inflammatory response and may therefore be an indicator for the degree of inflammation [1-4]. Lf originates in mucosal secretions, including tears, saliva, vaginal fluids, semen, nasal and bronchial secretions, bile, gastrointestinal fluids, urine and in milk and colostrum [5,6]. High levels of neutrophil-released Lf will prevent colonization, and thereby infection of host tissues, inhibit the growth of microorganisms, and protect tissues from damage. The prevention of damage is effectuated in different ways, such as iron binding to cause inaccessibility for oxygen radical production, binding low-density lipoprotein receptor-related protein 1 (LRP1) and promoting tissue repair, and binding lipopolysaccharides (LPS) and dampening LPS mediated inflammatory signaling [7]. Additional physiological functions of Lf include regulation of iron absorption in the bowel, antioxidant and anticarcinogenic properties [6]. Lf is produced in high amounts in breast milk, particularly in colostrum, which may cause increased levels of lf in the intestine in small children that are breast feed [6,8]. Studies have suggested protective effects of Lf against neonatal sepsis and necrotizing enterocolitis (NEC), an inflammatory gastrointestinal disorder affecting primarily preterm infants [9]. In particular, one RCT study conducted by Manzoni et al. [10] reported that bovine Lf supplementation significantly reduced the incidence of NEC and/or mortality compared with placebo [10]. In another RCT study conducted by Akin et al. [11], Lf prophylaxis significantly reduced nosocomial sepsis episodes and concurrently increased levels of T-regulatory cells in infants either VLBW or born before 32 weeks of gestation, in which the increased levels of T-regulatory cells were the proposed protective effect of Lf on nosocomial sepsis [11].
Lf is also produced in the pancreas in small amounts [12]. In our experience, Lf from the intestine and pancreas is small compared to the inflammation caused by bacterial intestinal infections. Lf seems to be a more reliable measure for inflammation than the counting of PMN’s in feces [13-15]. Acute gastroenteritis is a common and well-known illness by many, following its ability to affect people of all ages [16-20]. It refers to conditions of diarrhea and/or vomiting following a non-inflammatory infection in the upper small bowel or inflammatory infection in the colon. The etiology can be bacteria, virus or parasites, but in many cases no pathogen is identified [21]. Acute infectious diarrhea can be classified into two clinical groups commonly referred to as non-inflammatory with mostly viral etiology and a milder clinical disease, and inflammatory with mostly invasive or with toxin-producing bacteria, and a more severe course of clinical disease. Common pathogens in the non-inflammatory infectious diarrhea include Enterotoxigenic Escherichia coli (ETEC), Clostridium perfringens, Bacillus cereus, Staphylococcus aureus, Rotavirus, Norovirus, Giardia, Cryptosporidium, Vibrio cholera, while Salmonella (non-typhi spp.), Shigella spp, Campylobacter spp, Shiga toxin–producing E. coli (STEC), enteroinvasive E. coli (EIEC), Clostridium difficile, Entamoeba histolytica, and Yersinia are common pathogens of the inflammatory infectious diarrhea [22]. Enteric bacteria can cause disease by both toxigenic and invasive mechanisms. Pathogens such as Salmonella spp., Shigella spp, Campylobacter spp, enteropathogenic E. coli, Yersinia enterocolitica, Vibrio cholera, C. difficile and certain types of C. perfringens all produce toxins or adhere to the intestinal mucosa or both and thereby cause dysbiosis in the intestinal flora [23]. In particular, E.coli is known to cause diarrhea in several ways, owing to its number of pathotypes marked by either colonization mechanisms or specific toxin-production, such as verocytotoxinproducing E.coli (VTEC), also termed shiga toxin-producing E.coli (STEC), enteropathogenic E.coli (EPEC), enteroinvasive E.coli (EIEC), enterotoxigenic E.coli (ETEC), and enteroaggregative E.coli (EAEC) [24]. Among diarrhoegenic E. coli, STEC are the most virulent to date. STEC induced diarrhea can cause a range of symptoms in humans, starting from mild diarrhea to a more severe form such as haemorrhagic colitis and haemolytic-uraemic syndrome (HUS) [24]. Shigella sonnei is known to cause gastroenteritis characterized by bloody diarrhea, fever, and abdominal pain [25].
Purpose of this study
The aim of this study was to investigate the degree of inflammation measured by the level of feces Lf compared to the culture of well-known enteric pathogenic bacteria, and in toddlers to examine if the intake of breast milk was associated with higher levels of fecal Lf when compared to non-breastfed toddlers.
Material and methods
Fecal samples: Lf levels were measured in 512 consecutive fecal samples from patients and sent to the Department of Clinical Microbiology for detection of bacterial enteric pathogens. The patients were included when the department found it indicated to examine fecal samples for enteric pathogen bacteria which included symptoms of diarrhea. Twenty healthy volunteers around 18-20 years of age with no symptoms of gastroenteritis were also tested for fecal Lf, contributing as controls in our study.
Culture of well-known bacterial enteric pathogens: Fecal samples were cultured on selective media for Salmonella spp., Shigella spp, Yersinia enterocolitica. and Vibrio cholera. Salmonella spp. and Shigella spp. were identified by serologic testing. Lactose negative E. coli were tested for serologic type to identify pathogenic E. coli. Campylobacter spp. were identified by microaerobic culture at 370C and 420C on blood agar plates after filtration through a 0,45 μm filter for one hour. C. difficile were cultured anaerobic on CCFA plates and C. perfringens were treated with ethanol and cultured on 10% blood agar plates.
Measuring lf: Lf was measured by an in-house ELISA. In brief, a 96- Well Microtiter plate was coated with feces and washed. Antibodies to Lf was added and washed. The chromogenic reaction was visualized with the tetrameric biotin-binding protein avidin and the enzyme Horseradish peroxidase (HRP) and read in an ELISA reader.
Toddlers: Medical information on toddlers were obtained retrospectively from the National Diagnostic Register. Age at time of feces sampling, sex, degree of gastrointestinal symptoms as described in medical records, level of white blood cell count (WBC), level of C-reactive protein (CRP), whether or not they had consumed breast milk or formula milk, any prior surgical treatments, any prior diagnosis of interest including congenital conditions, and antibiotic treatment for the gastrointestinal symptoms and/or other medical indications at the time of fecal sampling were carefully recorded. The gastrointestinal symptoms were grouped in three categories: mild, moderate, and severe. Mild gastrointestinal symptoms were defined as less than three bowel movements a day, moderate gastrointestinal symptoms were defined as between three and eight bowel movements a day, and severe gastrointestinal symptoms were defined as either more than eight loose bowel movements in a single day, or watery and/or bloody bowel movements in a single day regardless of frequency.
Statistical analysis: For comparisons of the categorical data, the Chi-square test and Fisher’s exact test were used whenever applicable. Fisher’s exact test (two-sided) was used when cell counts were less than five [26,27]. The tests were used to determine if there is nonrandom associations between two categorical variables exemplified with following null hypothesis H0: a bacterial organism is not associated to higher levels of Lf (> 1,000 pg/ml) in feces when compared to normal young adults with no gastrointestinal symptoms and a fecal Lf level < 100 pg/ml [28]. P-values < .05 were considered statistically significant.
Results
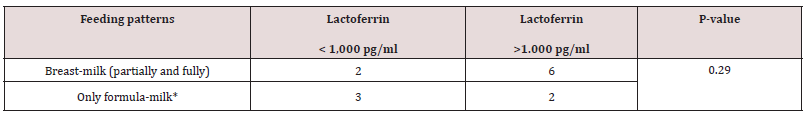
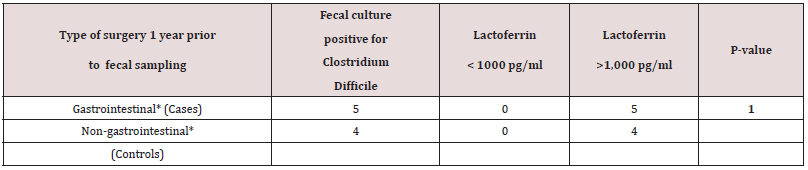
A total of 512 patients between 0 and 94 years of age underwent fecal cultures and fecal Lf measurement at Copenhagen University Hospital (Rigshospital). In addition, twenty young and healthy adults between 18-20 years of age also underwent fecal Lf measurement to serve as controls. Demographic data of 50 out of 84 toddlers between three weeks and less than three years of age who underwent feces sampling and fecal Lf measurement at Copenhagen University Hospital (Rigshospital) from January 2002 to March 2003 are listed in Table 1. For the remaining 34 toddlers, the 428 patients with an age between three and 94 years, and the twenty young adults serving as controls, demographic data, and GI symptomatology at time of fecal sampling were not available. Twenty-six (52%) out of 50 toddlers were fed breast milk, but only five of these toddlers were fed breast milk, exclusively (Table 1). Thirty-one (62 %) out of 50 toddlers were fed formula milk, and nine of these toddlers never had breast milk (Table 1). Breast-milk (partially and fully) vs formula-milk and fecal Lf levels in toddlers < 6 months of age revealed no significant differences (Table 1a). Nineteen (19%) out of 50 toddlers had surgery for conditions listed in Table 1, and some of these toddlers (n = 4) had surgery for more than one condition, but fecal sampling with Lf measurement was obtained with a range of 2 days to two years after surgery. Only three toddlers (16%) had their feces sampled within three days after surgery. Sixteen (84%) out of 19 toddlers with prior surgery of any kind to fecal sampling, had fecal Lf levels > 1,000 pg/ml, while nine (100%) out of nine toddlers with prior gastrointestinal surgery prior to fecal sampling, had fecal Lf levels >1,000 pg/ ml. These persistently high fecal Lf levels (>1,000 pg/ml) were matched for age (range 6-13 months), fecal culture and time (1 year) of fecal sampling after undergoing surgery and examined in an association-analysis (Table 1b). Results revealed no significant associations between higher fecal Lf levels (>1,000 pg/ml) a year after gastrointestinal surgery compared with non-gastrointestinal surgery (Table 1b).
Table 1: Demographic Data and selected Hospitalization Details of 50 children who underwent a fecal culture and fecal lf measurement between the year 2002 to 2003.

*Morbus Hirschprung, necrotizing enterocolitis, esophageal atresia, intestinal atresia, intestinal malrotation, volvulus and diaphragmatic hernia
†Infantile spasms (west syndrome), campomelic dysplasia, unilateral renal agenesis, congenital heart disease, acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML), aplastic anemia, cystic fibrosis, cholestasis groenlatica (liver disease), griscelli syndrome, degeneratio corticalis cerebri non specificata, wills tumor, bronchopulmonal dysplasia, hemoglobin deficiency, osteogenesis imperfecta, and congenital hip dislocation.
Abbreviations -> GA: gestational age, NEC: necrotizing enterocolitis.
Values expressed as no. of toddlers.
*Includes toddlers who never had breast-milk.
Values expressed as no. of toddlers.
Gastrointestinal surgery includes: morbus Hirschprung, necrotizing enterocolitis, esophageal atresia, intestinal atresia, intestinal malrotation, volvulus and diaphragmatic hernia.
Non-gastrointestinal surgery includes congenital heart diseases, campomelic dysplasia, congenital hip dislocation, ruptured congenital mesoblastic nephroma.
The highest of all fecal Lf level observed (>50,000 pg/ml), was from one male toddler who underwent fecal sampling two days after undergoing traumatic surgery of a fractured left femur. Seventeen (85%) normal adults had Lf levels <100 pg/ml in feces, three (15%) had levels between 100 and 1000 pg/ml, and none had Lf levels of 1,000-10,000 g/ml or > 10,000 pg/ml in feces (Tables 2 & 3). Therefore, it is assumed that a level <100 pg/ml is the normal level of Lf in feces in individuals without symptoms of gastroenteritis. Thus, a level < 100 pg/ml, 100-1,000 pg/ml, 1,000-10,000 g/ml and above 10,000 pg/ml were examined in this study (table 2 and 3). In 395 (93%) culture positive patients between three and 94 years of age listed in Table 2, C. difficile 216 (54%) was detected most commonly in feces followed by Campylobacter jejuni/coli 49 (11%). All bacteria-positive feces cultured, apart from E. coli (STEC), and fecal Lf levels > 1,000 pg/ml were significantly associated when compared with controls with fecal Lf levels <100 pg/ml (p <.05) (Table 2). Even though the numbers were small, all S. sonnii and E. coli (STEC) (100%) indicated severe inflammation with fecal Lf levels > 10.000 pg/ml, although only S. sonnii revealed a significant association when compared to normal young adults with Lf levels < 100 pg/ml in feces (p= .0002), while E. coli (STEC) did not (p= .056) (Table 2). Salmonella enteritidis indicated high inflammation with the majority having Lf levels >1,000 pg/ml (78%), whereas atypical Campylobacter (C. uppsaliensis, C. concisus) indicated less inflammation with the majority of patients having fecal Lf levels < 1,000 pg/ml (46 vs 43%) (Table 2). More than 40% of patients with diarrhea had fecal Lf levels > 1,000 pg/ml even if no enteric pathogenic bacteria were cultured and when compared with controls, results were significant (p < .0001) (Table 2). In 82 (98%) fecal culture positive toddlers less than three years of age listed in Table 3, C. difficile 47 (56%) was detected most commonly followed by C. perfringens (enterotoxin negative) (13%). In toddlers, C.
difficile indicated less inflammation with the majority having fecal Lf levels 100-1,000 pg/ml (51% vs 45 %) (Table 3). Even though the numbers were small, all Campylobacter jejuni/coli indicated high inflammation with fecal Lf levels > 1,000 pg/ml (Table 3). E. coli (EPEC) (75%) and Salmonella spp. (75%) also indicated high inflammation with the majority of toddlers having fecal Lf levels > 1,000 pg/ml (Table 3). All bacteria-positive feces cultured, apart from Salmonella enteritidis and C. perfringens (enterotoxin positive), were significantly associated with fecal Lf levels > 1,000 pg/ml when compared with controls with fecal Lf levels <100 pg/ml (p <.05) (Table 3). A gradual division of gastrointestinal symptoms in relation to levels of fecal Lf in 50 toddlers less than three years of age was noted in Table 4. Most toddlers had mild gastrointestinal symptoms (n=26), followed by severe (n=11), and moderate (n=5). Fisher’s exact test on a 3x4 contingency table revealed insignificant associations between the degree of gastrointestinal symptoms and the levels of fecal Lf (p-value 1.0).
Table 2: The bacteriologic culture and fecal Lf levels in patients between three and 94 years of age.

Values are expressed as No. (%)
P-values marked with bold indicate statistically significant p-value at a significance level of 0.05.
Table 3: The bacteriologic culture and fecal lf levels in children between three weeks and less than three years of age.
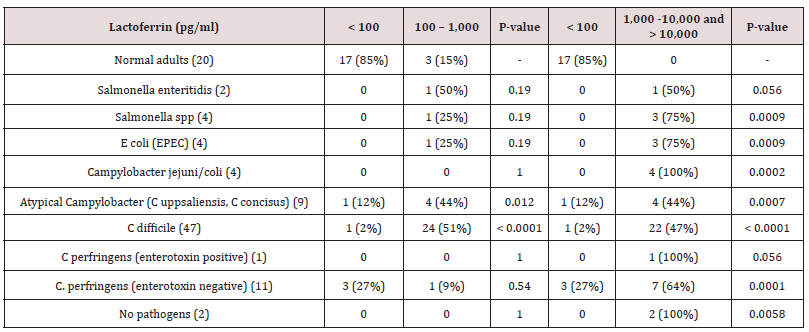
Values are expressed as No. (%).
P-values marked with bold indicate statistically significant p-value at a significance level of 0.05.
Table 4: Degree of gastrointestinal symptoms compared with different fecal Lf levels in children between three weeks and just below three years of age.
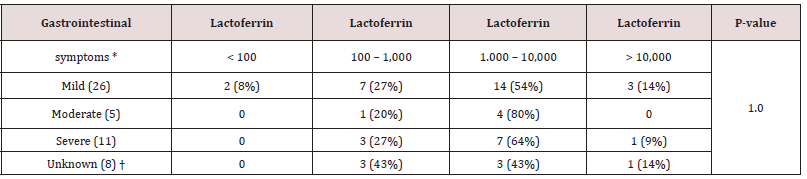
Lactoferrin values expressed as pg/ml.
*Values are expressed as No (%).
†Medical records of the diarrhea not available.
Discussion
Our aim of study was to compare levels of fecal Lf to the culture of well-known enteric pathogenic bacteria in both toddlers and adults, and in toddlers to examine if the intake of breast milk was associated with higher levels of fecal Lf when compared with nonbreast fed toddlers. Interestingly, the majority of children with a history of gastrointestinal surgery had persistently high fecal Lf levels > 1,000 pg/ml even one year after undergoing surgery compared with non-gastrointestinal surgery, but no significant association between gastrointestinal surgery and persistently higher fecal Lf levels after matching for age, fecal culture and time after undergoing surgery were observed in our study. Another interesting observation was one toddler with severe trauma to the entire body and subsequently orthopedic surgery who had fecal Lf levels > 50,000 pg/ml two days after surgery which was the highest level represented in this study. In theory, it seems likely that these observations of high Lf levels may depict the inflammatory response related to surgery and to trauma, however assessment of an association analysis concerning trauma was not possible since no more than one case of trauma surgery was observed. In consistency with expected [29,30], infection with S. sonnei and E. coli (STEC) were associated with the highest fecal Lf levels >10,000 pg/ml in patients between three and 94 years of age indicating severe inflammation, though not significant due to small numbers of positive cultures with the respective bacteria, in our study. A similar pattern was observed in toddlers, where levels of Lf >1,000 pg/ml in all bacteria-positive feces revealed associations of significance when compared with fecal Lf levels <100 pg/ml in normal young adults indicating high inflammation, though not significant when concerning Salmonella enteritidis and C. perfringens (enterotoxin positive) due to small numbers of positive cultures with the respective bacteria, in our study. According to theory [31,32], it would be expected that the presence of an enterotoxin would cause a higher degree of inflammation, and thus a higher level of fecal Lf in comparison to the absence of one, but surprisingly this was not observed in our study. Interestingly, culture-negative results in more than 40% of patients between three and 94 years of age and in toddlers less < 3 years of age with gastrointestinal symptoms revealed levels of fecal Lf >1,000 pg/ml indicating high inflammation without the presence of a positive fecal culture. Possible, this could be explained by the fact, that the presence of other pathogens, such as viruses, parasites, and other non-well known enteric bacterial spp. as well as non-infectious causes of diarrhea, were not examined. Noninfectious causes of diarrhea include medication adverse effects, acute abdominal processes, gastroenterologic disease, and endocrine disease [22]. Since we did not obtain any medical records of the patients more than three years of age in our study, any of the above mentioned reasons could in theory account for diarrhea in these culture negative patients. Contrary to our expectations, fecal Lf levels were independent of the severity of gastrointestinal symptoms in 50 toddlers, possible due to a small number of sample size. Since Lf in breastmilk is high and contains more than 5 g/L in colostrum and later decreases to 2–3 g/L in mature milk [33], we investigated if breastfed toddlers until six months of age, had higher levels of fecal Lf compared to toddlers of same age that never had breast milk, and surprisingly they did not.
This study has some strengths and limitations. Strengths include its large fecal sample size which is more representative of the population. An important limitation to our study was the fact that fecal Lf levels in toddlers were compared to Lf levels of normal young adults at the age of 18-20, thus not being age-, gender-, or race-matched which indeed cannot account for potential and underlying confounders. Further, we did not examine fecal cultures for other pathogens than listed in our tables as mentioned above, and controls did not have their feces cultured. Another important limitation to our study was the fact that we did not have medical records of the majority of the patients or the controls that provided a fecal sample, thus not being able to match them. Furthermore, medical records of the gastrointestinal symptoms were information of which the attending physician obtained through anamnesis predominantly from family, and this type of data collection can undoubtedly be subject to information bias. Also, the association analysis between gastrointestinal surgery and persistently higher fecal Lf levels after matching resulted in a small sample size, which may have prevented the findings from being extrapolated. Lastly, the precise amounts of breast vs formula milk intake in toddlers were not known and a preferred analysis of cases matched to controls based on gender-, fecal culture-, and race was not possible, due to a small number of cases with available records of feeding patterns, making it difficult to find enough matches for the analysis to produce reliable results. Previous research demonstrated fecal Lf to be a noninvasive diagnostic marker when evaluating the severity of intestinal inflammation in patients presenting abdominal pain and diarrhea[34-36] and in chronic inflammatory bowel diseases [35,37-39]. All of these studies suggest a potential use of fecal Lf as a marker of inflammatory disease, and the same use could be investigated concerning neonatal necrotizing enterocolitis. Moving forward, we advise future research in this area to construct a study in which fecal Lf is measured at onset, during and after treatment of NEC patients. In conclusion, bacteria-positive fecal cultures were significantly associated to higher levels of fecal Lf when compared with normal, young and healthy men and women with normal fecal Lf levels, and the more aggressive the bacteria cultured was, the higher level of fecal Lf was observed in our study. The severity of gastrointestinal symptoms described in the 50 toddlers < 3 years of age were independent of fecal Lf levels in our study. In an unmatched association-analysis, no significant differences between breast milk and formula milk concerning fecal Lf levels were observed in our study.
For more Lupine Publishers Open Access Journals Please visit our website:
https://lupinepublishers.blogspot.com/
For more journal paediatrics and neonatology articles Please Click Here:


No comments:
Post a Comment